A fundamental goal of cardiac research is to describe fully the motion of the heart wall during the cardiac cycle.
Introduction
A technique for isolating individual kinematic modes in left ventricular myocardium is presented using MRI cardiac tagging data and displacement field fitting[1] to a prolate spheroidal harmonic series. Previous researchers have fit LV motion to up to 7 global deformation modes[2,3]. Though enlightening, this approach imposed on the motion a priori kinematic assumptions and limited degrees of freedom.
Methods
Three-dimensional (3D) MRI tagging data sets were acquired in human volunteers and cardiac patients, giving informed consent, using a 1.5T scanner and a cine breath-hold imaging sequence with parallel tags[4]. A high density 3D tagging data set designed to oversample the LV motion was acquired in one volunteer(Fig. 1). The resulting 7,600 samples of tag point displacement were used to compute the individual contribution of 324 candidate kinematic modes (plus the 6 bulk motions). The modes with the largest contributions to normal LV systolic deformation were identified and their motions demonstrated on a computer-generated heart model. Conventional tagging and motion reconstruction protocols (150 modes) were used on a group of 18 additional volunteers and 12 cardiac patients. Of the 12 subjects in the patient group, 4 were determined to have posterior wall functional deficits, as determined by analysis of their 3D strain maps, and this subgroup was used for comparison. The significance of the differences in mode contributions were determined using Mann-Whitney statistics.
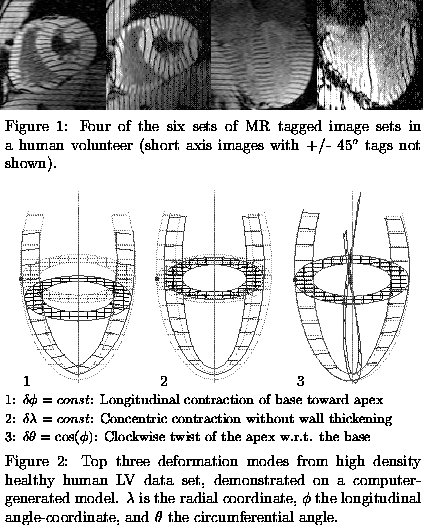
Longitudinal contraction modes comprised two of the top five contributors in healthy hearts (Fig. 2), and a significant decrease in these modes was found in cardiac patients exhibiting left ventricular posterior wall functional deficit. In all, 17 modes showed significant (P<0.05) changes in contribution between this patient group and the volunteer group. Notably absent from the major modes in healthy volunteers were modes contributing to transmural gradients in motion, yet significant alterations were found in 5 such modes in the patient group.
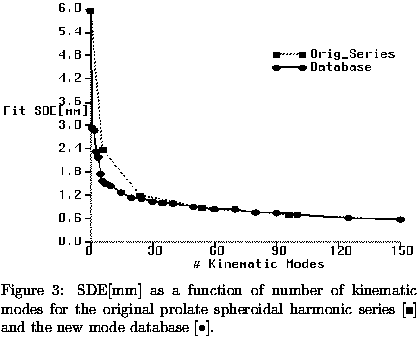
These results demonstrate that for clinical diagnosis based upon global modes of motion, measurement of longitudinal displacement of the LV may be critical, and thus an increased emphasis on acquiring sufficient long axis image data may be warrented. They also suggest that methods sensitive to transmural differences in displacement may offer a clinical advantage for diagnosis of functional abnormality. Finally, the creation of a kinematic mode database can greatly increase reconstruction efficiency in healthy hearts by eliminating modes which contribute little to the reconstruction accuracy.
References
1. O'Dell WG, et al, Radiology, June, 1995.
2. Arts T, et al, Journal of Biomechanics, 25:10, 1992
3. Buchalter MB, et al, Circulation, 81, 1990
4. McVeigh ER and Atalar E, Magn Reson in Med, 28, 1992
Acknowledgements
This research was funded by NIH grants HL45090 and HL45683. E.R. McVeigh
is an Established Investigator of the American Heart Association. W.G. O'Dell
is funded by American Heart Assoc. Post-Doctoral Fellowship.